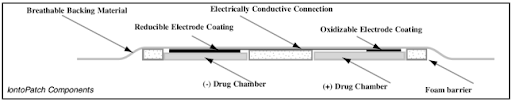
IontoPatch™ Technical Overview
IontoPatch™ products are intended to be used for the administration of soluble salts into the body for medical purposes as an alternative to hypodermic injection. Products are intended to be used in situations when it is desirable to avoid the pain or danger that may be imposed by needle insertion and injection.
For skin safety, IontoPatch™ products have been designed for use with low levels of direct current and are designed to administer medication slowly and continuously over several hours.
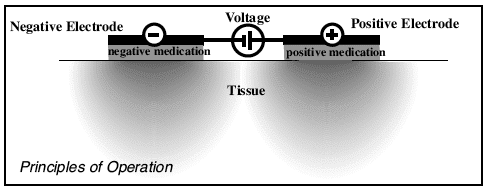
Principles of Operation
Iontophoresis is a process that utilizes bipolar electric fields to propel molecules across intact skin into underlying tissue. Positively charged ions in solution are transferred from a positive (+) chamber, while negatively charged ions in solution are transferred from a negative (-) chamber. Ions are transferred to the body at a rate proportional to the magnitude of the current flow between electrodes.
The total number of molecules transferred during iontophoresis is related to the total electric charge utilized; one equivalent weight of molecules is delivered by one Faraday of charge (96,500 coulombs, where one coulomb = 1 amp-second). Conventional units of delivery dosage in iontophoresis are milliamp-minutes, which are calculated as [milliamps of current applied] x [time of application in minutes].
Iontophoresis is now commonly used by caregivers to deliver water-soluble anti-inflammatory medications locally into sub-acute or acute inflammations, as an alternative to syringe-and-needle injection (ref. 1). Studies have shown iontophoresis has penetrated medication to depths of at least 1 cm (ref. 1).
Iontopatch Design
Iontopatch products are disposable single-use devices, with a self-contained power source. Both the negative and positive chambers are contained within the Iontopatch drug delivery system. In use, the Iontopatch can simultaneously deliver negatively and positively charged compounds by placing each compound in the relevant chamber.
A cross-section of a representative Iontopatch is shown below.
Iontopatch Coatings
Coatings on the Iontopatch electrodes provide a potential of approximately one volt, which induces a current flow when the patch is applied to the body. An integrated 3V battery may provide additional potential to give a total potential of four volts.
Current flow is induced when the patch is applied to the body. Electrode coatings are gradually consumed during use, and current flow is suspended when the coatings are depleted. Precisely known amounts of coatings are deposited on the electrode surfaces during the manufacturing process.
On the electrodes:
A zinc coating is oxidized at the positive (anodic) electrode surface:
Zn = Zn(++) + 2e(-) E° = -0.76
A silver chloride coating is reduced at the negative (cathodic) electrode surface:
AgCl + e(-) = Ag + Cl(-) E° = 0.22
Δ E° = 0.98 volts
Patch-to-Patch Reproducibility
For a typical manufactured Iontopatch lot, electrode capacity is determined by immersing the electrode in a 10% NaCl solution and discharging it against a counter-electrode with a 10-Ohm resistor placed in series within the circuit. Current flow is recorded until it falls to 1% of the peak value.
Patch-to-Patch Reproducibility: Test Results
Number of Patches Tested: 200
Labeled Capacity: 80 mA-min
Measured Capacity mean: 81.4 mA-min
Measured Capacity sd: 3.8 mA-min
Measured Capacity cv: 4.7%
Delivery Rate: Theoretical considerations
With Iontopatch products, the power source potential will induce a current flow between electrode chambers according to Ohm’s Law:
V = IR; where V = voltage (volts), I = current (amps), and R = resistance (ohms)
The electrical properties of skin are complex and most biological systems are nonlinear and therefore change with time (ref 2). Consequently, individual skin resistance and delivery rate for the Iontopatch will vary and are dependent on factors such as skin cleanliness, skin hydration, and the number of sweat pores at the delivery site.
IontoPatch™ Delivery Time
Delivery time is dependent upon individual patient skin resistance. For the average patient, dosage delivery for the Iontopatch 80 and SP products will be complete in approximately 14 hours and the rate of drug delivery will be substantially reduced after 24 hours.
For the average patient, dosage delivery for the Iontopatch STAT® product will be complete in approximately 4 hours and the rate of drug delivery will be substantially reduced after 6 hours.
For the average patient, dosage delivery for the Iontopatch Extra Strength™ product will be complete in approximately 8 hours and the rate of drug delivery will be substantially reduced after 12 hours.
Delivery Rate: Test Results
Iontopatches built to 40 mA-min capacity were loaded with 1% NaCl solution and placed on differing locations on human subjects. Current flow was monitored over the course of 4 hours, with the following results:
Number of Iontopatches tested: 8
Average Current Flow: 0.09 mA
Range: 0.05-0.16 mA
From this data, the average current flow for Iontopatch STAT®, which has a potential of four volts, is estimated to be 0.36 mA. From this data, the average current flow for Iontopatch Extra Strength™, which has a potential of four volts and a series resistance of approximately 4kOhms, is estimated to be 0.26 mA.
Delivery Amount: Theoretical Considerations
The charge transferred during iontophoresis can be attributed to many factors, including delivery of soluble ions intended, delivery of ‘competing’ ions of similar charge, and oppositely charged ions moving in reverse direction. Delivery efficiency can be defined as the percentage of current flow attributable to the ion intended to be delivered.
Delivery Amount: Test Results
Delivery of a model negatively charged compound was evaluated using both in-vitro (laboratory) and in-vivo (on human volunteer subjects) conditions.
In both cases, iontophoretic delivery was determined by subtracting passive delivery (e.g. transfer in the absence of current flow) from total delivery (transfer in the presence of current flow).
In-Vitro & In-Vivo Delivery Testing
In-Vitro Delivery: In-vitro testing was conducted using glass diffusion cells, with battery electrodes immersed in the receiver and donor compartments separated by an ultrafiltration membrane. Total charge transfer was limited to 80 mA-minutes and was measured by monitoring current flow with a data acquisition station connected in line with the batteries.
In-Vivo Delivery: In-vivo delivery was determined by loading 80 mA-min Iontopatches with an exactly known amount of model compound, placing them on volunteer subjects for 24 hours, and measuring the residual content of the model compound by extraction from the patch after removal from the subjects.
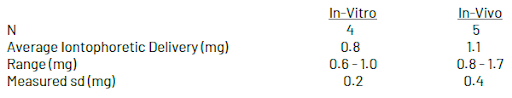
Warnings and Precautions
Use all appropriate precautions related to the compound intended for delivery. Acetic Acid (the non-ionic acid form of Sodium Acetate) and Iodine (the non-ionic form of Iodide) are not recommended for use with the Iontopatch. Since Acetic Acid and Iodine are non-ionic, they will not be delivered by iontophoresis and they may cause skin irritation.
Iontopatch Contraindication
A contraindication is a situation in which a medicine or procedure should not be used because it may be harmful to the patient. Iontopatch is contraindicated for use over damaged or denuded skin, and for treatment around the orbital region of the head.
Patient Precautions
- Patients should be asked about their history of drug allergies or sensitivities.
- Patients should be advised to remove the lontoPatch STAT after 6 hours.
- Patients should be advised to report any undue burning or pain at once during treatment. The treatment should be paused, the area under the electrodes should be inspected, and any necessary corrective action should be taken before resuming treatment.
- Do not wrap tightly or apply excessive pressure for long periods of time.
Removing Iontopatch
To minimize skin irritation, patients should be instructed to remove the patch slowly using soap and water in the shower or bath.
Patient Warnings
Potential systemic adverse effects may result from the use of this device. Drugs or solutions delivered with this device have the potential to reach the bloodstream and cause systemic effects.
Carefully read all labeling of the drug or solution used with this device to understand all potential adverse effects and to ensure appropriate dosing information. If systemic manifestations occur, refer to the drug or solution labeling for appropriate action.
Iontophoresis can cause skin irritation and burns, including redness under the pads. Skin discoloration or hyper-pigmentation, caused by the adhesives and/or some medications, is possible. These conditions will generally resolve over time, once iontophoresis is discontinued.
Iontopatch products should not be worn during MRI procedures.
pH and Current Density Effects: Theoretical Considerations
Iontophoretic systems, when improperly administered, have been known to cause skin damage and pain (ref 3). With many conventional iontophoresis systems, inert carbon, platinum, or gold electrodes are used, which result in the electrolysis of water at the electrode surface as current flows. Water electrolysis produces hydrogen gas and high pH at the cathode, and oxygen gas and low pH at the anode. These pH changes and high current densities are considered the cause of skin injury associated with iontophoretic treatment (ref 1).
Buffers are sometimes added to iontophoretic patches to mitigate pH change, but these salts compete with medication delivery and reduce delivery efficiency. The Iontopatch electrodes do not electrolyze water, require no buffer salts, and use current densities that are below those associated with skin damage (ref 3).
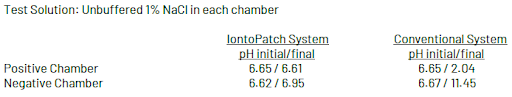
pH Change With Use of the Iontopatch: Test Results
To test the effect of Iontopatch electrodes and battery discharge on the pH of solutions contained in the patch, an in-vitro apparatus was set up with anionic and cationic chambers separated by a 1 M KCl agarose salt bridge.
pH before and after battery discharge of a 40 mA-min patch was measured using a pH meter calibrated with NIST traceable standards. For comparison purposes, the same apparatus and unbuffered solutions were tested using a galvanostat and conventional carbon electrodes.
Current Density: Test Results
High current density levels are also considered as a cause for skin damage, with recommended current densities to be below 1 mA per square inch to avoid harmful effects (ref 1,3). Iontopatches built to 40 mAmin capacity were loaded with 1% NaCl solution and placed on differing locations on human subjects. The highest current density measured was compared to the maximum current densities recommended in literature references (ref 1,3). The expected maximum current densities for the Iontopatch STAT and Iontopatch Extra Strength are calculated from the Iontopatch study.
![]()
References
- C. Costello, A. Jeske: Iontophoresis: Applications in Transdermal Medications Delivery. Physical Therapy 75: 104-113,1995.
- P. Prausnitz: The effects of electrical current applied to skin: A review for transdermal drug delivery. Advanced Drug Delivery Reviews 18: 395-425,1996.
- L. Li, R. Scudds: Iontophoresis: An Overview of the Mechanisms and Clinical Application. Arthritis Care and Research 8: 51-61,1995.